临床试验设计软件
Instructions: To access the software online click the red circle or the title. To download a desktop version, click the download arrow. To expand software description, mouse over the description.
注: 本网站上线软件正在不断更新中,部分功能不完善还待更新,如遇相关技术问题,请及时联系我们。
BOIN Suite
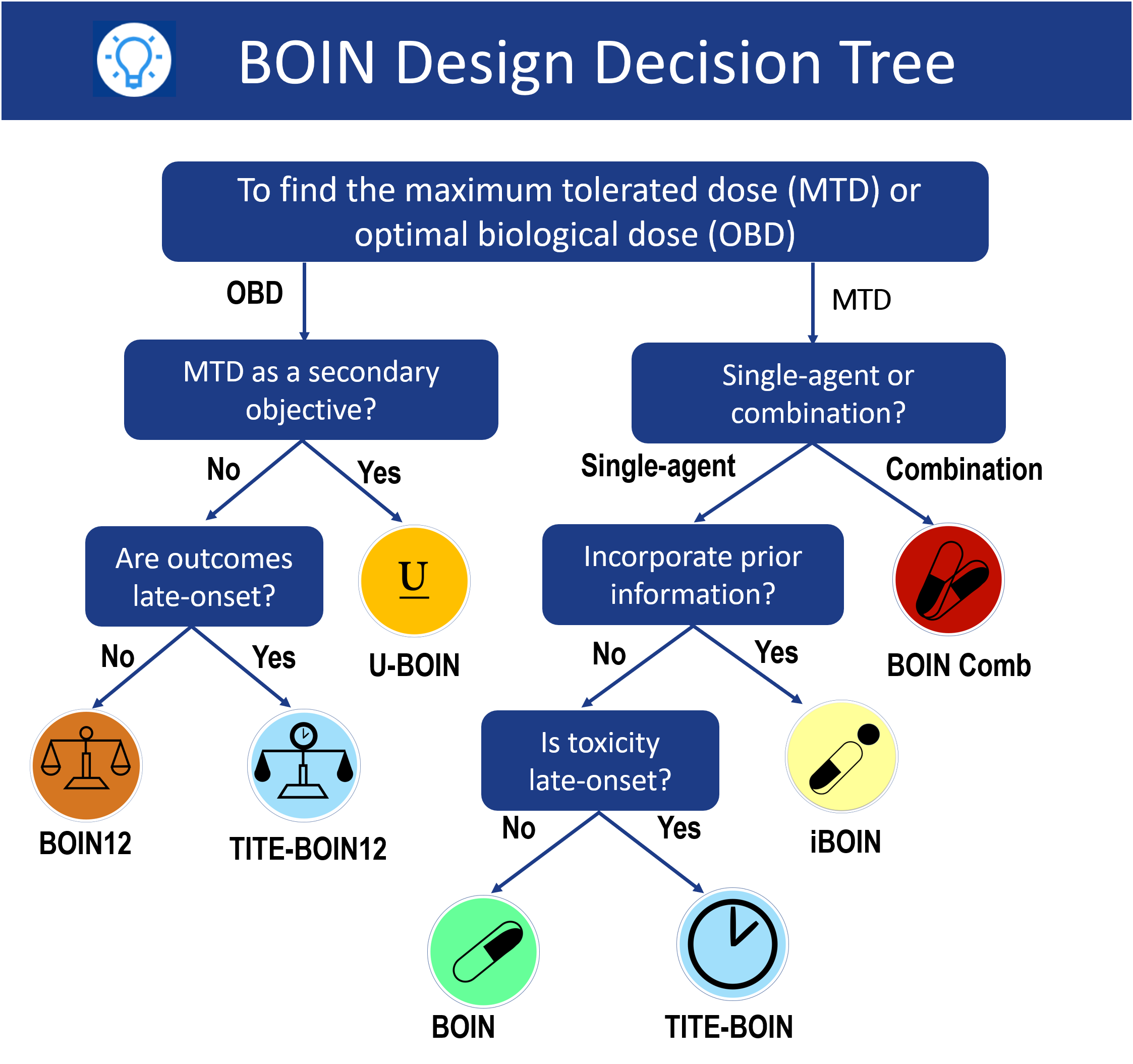
How to choose a design?
Bayesian optimal interval (BOIN) designs provide a novel platform to design phase I trials with single agent, drug combination, platform more...
CRM
& BMA-CRM
The continual reassessment method (CRM) is a model-based
dose-finding approach that assumes a parametric model for the dose-toxicity more...
Keyboard Suite
Keyboard designs provide a novel platform to design
phase I trials with single agent and drug combination. As model-assisted designs, the more...
Simon's
Two Stage Design
The Simon's two stage design is a commonly used phase II design.
It controlls type 1 more...
BOP2 Suite
BOP2 designs provide a Bayesian optimal platform to design phase II clinical trials with more...
Bayesian
Phase 2 Design with Delayed Outcomes
One practical impediment in adaptive phase II trials is that
outcomes
must be observed soon enough
more...
BOIN12: to find optimal biological dose for targeted and immune therapies
BOIN12 is a simple and flexible Bayesian optimal interval
more...
TITE-BOIN12: extension of BOIN12 for late-onset toxicity and efficacy
+ is an extension of BOIN12 to accommodate
more...
U-BOIN: a 2-stage design to find optimal biological dose for targeted and immune therapies
U-BOIN is a utility-based seamless Bayesian phase I/II trial
more...
Isotonic regression design to find optimal biological dose
This design is used to find the optimal biological dose (OBD) for
molecularly targeted agents and
more...
Calibrated Bayesian Hierarchical Model Design
Bayesian hierarchical modeling has been proposed to adaptively
borrow
information across cancer
more...
Bayesian Latent Subgroup Design for Basket Trials
The innovation of the BLAST design is that it adaptively
clusters
cancer types within a basket
more...
Bayesian Drug Combination Platform Trial Design with Adaptive Shrinkage
ComPAS provides a flexible Bayesian platform design to
efficiently screen a large number of drug
more...
Binary
Outcome
Includes equality, equivalence, superiority, and
more...
Continuous
Outcome
Includes equality, equivalence, superiority, and
more...
Time
to Event Outcome
Includes tests under exponential assumption for:
more...
Group Sequential Outcome
Includes tests for Two Means, Two Proportions, and Survival Endpoints:
more...
质量控制
本网站程序采用严格的软件工业标准开发和质量控制
- 源代码经多名软件工程师和临床试验专家审查,以确保其准确性、完整性、可重复性和稳健性。
- 软件在发布前已在真实世界研究中的极端案例下进行广泛的压力测试。
- 接受世界各地过千名用户和专家的反馈,持续更新软件。
- 使用Microsoft Visual Studio等工具确保语法准确无误。
- 使用Microsoft Team Foundation Server源代码控制系统维护和管理文件,记录和追踪文件改动。
- 使用成熟的软件开发技术,确保应用程序准确性、稳健性和可重复性。
Selected Publications
(* 指导的博士生或博士后)
-
Clinical Trial Design
- Jun, Yin. and Yuan, Y. (2020) Checkerboard: A Bayesian Efficacy and Toxicity Interval Design for Phase I/II Dose-finding Trials, Journal of Biopharmaceutical Statistics, in press.
- Jin IH, Zhang Z, Yuan, Y. (2020) Social Network Mediation Analysis: a Latent Space Approach, Psychometrika, in press.
- Zhou, Y., Li, R., Kuo, Y., Lee, JJ. and Yuan, Y. (2020) BOIN Suite: a software platform to design and implement novel early phase clinical trials, JCO Clinical Cancer Informatics, to appear.
- Zhou, Y., Li, R., Yan, F., Lee, JJ. and Yuan, Y. (2020) A comparative study of Bayesian optimal interval (BOIN) design with interval 3+3 (i3+3) design for phase I oncology dose-finding trials, Statistics in Biopharmaceutical Research , in press.
- Lin, R., Yan, F., Li, D. and Yuan, Y. (2020) BOIN12: Bayesian Optimal Interval Phase I/II Trial Design for Utility-Based Dose Finding in Immunotherapy and Targeted Therapies, JCO precision oncology , to appear.
- Lin, R., Yin, G., Yuan, Y., and Yang, Z. (2020) Sample size re-estimation in adaptive enrichment design, Contemporary Clinical Trials, to appear.
- Lin, R.*, Thall, P., and Yuan, Y. (2020) BAGS: A Bayesian Group Sequential Clinical Trial Design with Adaptive Subgroup-Specific Survival Time Comparisons, Journal of the American Statistical Association , in press.
- Li, Y., Yuan, Y. (2020) PA-CRM: A Continuous Reassessment Method for Pediatric Phase I Oncology Trials with Concurrent Adult Trials, Biometrics, to appear.
- Pan, H., Cheng, C. and Yuan, Y. (2020) Bayesian adaptive linearization method for phase I drug combination trials with dimension reduction, Pharmaceutical Statistics, in press.
- Lin, R., Thall, P. and Yuan, Y. (2020) A Phase III Basket Trial Design to Optimize Dose-Schedule Regimes Based on Delayed Outcomes, Bayesian Analysis, in press.
- Pan, H., Lin, R., Zhou, Y. and Yuan, Y. (2020) Keyboard Design for Phase I Drug-Combination Trials, Contemporary Clinical Trials, 92:105972.
- Zho,u H., Chen, C., Sun, L. and Yuan, Y. (2020) Bayesian Optimal Phase II Clinical Trial Design with Time-to-event endpoint, Pharmaceutical Statistics, 19, 776-786.
- Han, Y., Yuan, Y., Cao, S., Li, M. and Zang, Y. (2020) On the Use of Marker Strategy Design to Detect Predictive Marker E.ect in Cancer Immunotherapy, Statistics in Biosciences, 12, 180195.
- Lin, R.*, Yuan, Y. and Thall, P. (2020) An Adaptive Trial Design to Optimize DoseSchedule Regimes with Delayed Outcomes, Biometrics, 76(1):304-315.
- Lin, R.*, Coleman R. and Yuan, Y. (2020) TOP: Time-to-Event Bayesian Optimal Phase II Trial Design for Cancer Immunotherapy, Journal of the National Cancer Institute, 112(1):38-45.
- Lin, R.* and Yuan, Y. (2020) Time-to-Event Model-Assisted Designs for Dose-Finding Trials with Delayed Toxicity, Biostatistics, 21(4):807-824.
- Yan F., Pan H., Zhang L., Liu S. and Yuan, Y. (2020) BOIN: An R Package for Designing Single- Agent and Drug-Combination Dose-Finding Trials Using Bayesian Optimal Interval Designs, Journal of Statistical Software , 94(13):1-32. doi:10.18637/jss.v094.i13.
- Lee JJ , Yin G. (2020) Principles and Reporting of Bayesian Trials, Journal of Thoracic Oncology, https://doi.org/10.1016/j.jtho.2020.10.010.
- Xu G, Zhu H, Lee JJ . (2020) Borrowing Strength and Borrowing Index for Bayesian Hierarchical Models. Comput Stat Data Anal 144, 4/2020. PMCID: PMC7185234.
- Chen N, Lee JJ . (2020) Bayesian cluster hierarchical model for subgroup borrowing in the design and analysis of basket trials with binary endpoints. Stat Methods Med Res : 2020 Sep;29(9):2717-2732. PMID: 32178585.
- Yuan Y, Lee JJ , Hilsenbeck SG. (2019) Model-Assisted Designs for Early-Phase Clinical Trials: Simplicity Meets Superiority. JCO Precision Oncology 3:1-12.
- Ruberg, SJ, Harrell, FE, Jr., Gamalo-Siebers, M., LaVange, L., Lee, JJ, Price, K., Peck, C. (2019) Inference and Decision Making for 21st-Century Drug Development and Approval. American Statistician 73(sup1):319-327.
- Chen N, Lee JJ. (2019) Bayesian hierarchical classification and information sharing for clinical trials with subgroups and binary outcomes. Biom J 61(5):1219-1231, 2019. PMID: 30506747.
- Zhou Y, Lee JJ and Yuan Y. (2019) A utility-based Bayesian optimal interval (U-BOIN) phase I/II design to identify the optimal biological dose for targeted and immune therapies. Stat Med 38(28):5299-5316, 12/2019. e-Pub 10/2019. PMID: 31621952.
- Zhu H, Piao J, Lee JJ , Hu F, Zhang L. (2019) Response adaptive randomization procedures in seamless phase II/III clinical trials. J Biopharm Stat 30(1):3-17. e-Pub 8/2019. PMID: 31454295.
- Slack, R.S., Peng, A., Chen, M., Liu, D., Yuan, Y. and Lee J.J. (2019) Bayesian Clinical Trials at the University of Texas MD Anderson Cancer Center: An Update, Clinical Trials, 16(6):645-656, PMID: 31450957.
- Yan, F., Thall, P. and Yuan, Y. (2019) One-Year Outcomes after PCI Strategies in Cardiogenic Shock, The New England Journal of Medicine, 380:1876-1877.
- Lin R.* and Yuan, Y. (2019) On the Relative Efficiency of Model-Assisted Designs: A Conditional Approach, Journal of Biopharmaceutical Statistics, 29(4):648-662.
- Huang J.*, Yuan, Y. and Wetter D. (2019) Latent Class Dynamic Mediation Model with Application to Smoking Cessation Data, Psychometrika, 84(1):1-18.
- Tang, R., Shen, J. and Yuan, Y. (2019) ComPAS: A Bayesian Drug-Combination Platform Design with Adaptive Shrinkage, Statistis in Medicine, 38(7):1120-1134.
- Ursino, M., Yuan, Y., Comets, E., Favrais, G., Friede, T., Lentz, F., Stallard, N., Zohar, S. (2019) A dose-finding design for seizure reduction in neonates. Journal of the Royal Statistical Society: Series C , 68 (2):427-444.
- Shin, S.*, Yuan, Y., Strong, L.C., Bojadzieva, J. and Wang, W. (2019) Bayesian Semiparametric Estimation of Cancer-specific Age-at-onset Penetrance with Application to Li-Fraumeni Syndrome. Journal of the American Statistical Association, 114(526):541-552.
- Ahn, J.*, Morita, S.,Wang W. and Yuan, Y. (2019) Bayesian Shared Parameter Models for Dyadic Longitudinal Data with Informative Missing Data. Statistical Methods in Medical Research , 28(1):70- 83.
- Hobbs BP, Chen N, Lee JJ. (2018) Controlled multi-arm platform design using predictive probability. Stat Methods Med Res 27(1):65-78. e-Pub 1/2016. PMCID: PMC5039108.
- Du Y, Cook JD, Lee JJ. (2018) Comparing three regularization methods to avoid extreme allocation probability in response-adaptive randomization. J Biopharm Stat. 28(2):1-11, e-Pub 3/2017. PMID: 28323532.
- Yin, G., Chen, N. and Lee, JJ. (2018) Bayesian Adaptive Randomization and Trial Monitoring with Predictive Probability for Time-to-Event Endpoint. Statistics in Biosciences 10(2):420-438.
- Mu, R., Yuan, Y., Xu, J. , Mandrekar, SJ., and Jun Y. (2019) gBOIN: A unified phase I trial design accounting for toxicity grades. Journal of the Royal Statistical Society: Series C, 68, 289-308.
- Pan, H*., Liu, S., Miao, D. and Yuan, Y. (2018) Sample size determination for mediation analysis of longitudinal data. BMC Medical Research Methodology, 18(1):32. doi: 10.1186/s12874-018-0473-2.
- Guo, B., Li, D. and Yuan, Y. (2018) SPIRIT: A Seamless Phase I/II Randomized Design for Immunotherapy Trials. Pharmaceutical Statistics, 17, 527-540.
- Zhou, H.*, Yuan, Y., and Nie, L. (2018) Accuracy, Safety and Reliability of Novel Phase I Trial Designs. Clinical Cancer Research, 24, 4357-4364.
- Yuan, Y., Lin, R., Li, D., Nie, L., and Warren K. (2018) Time-to-event Bayesian Optimal Interval Design to Accelerate Phase I Trials. Clinical Cancer Research, 24, 4921-4930.
- Zhou, H.*, Murray, T., Pan, H. and Yuan, Y. (2018) Comparative review of novel model-assisted designs for phase I clinical trials. Statistics in Medicine, 37, 2208-2222.
- Yan, F. Thall, PF., Lu, KH., Gilbert, MR., and Yuan, Y. (2018) Phase I-II clinical trial design: A state-of-the-art paradigm for dose finding, Annals of Oncology, 29, 694-699.
- Chu, Y.* and Yuan, Y. (2018) BLAST: Bayesian Latent Subgroup Design for Basket Trials Accounting for Patient Heterogeneity, Journal of the Royal Statistical Society: Series C, 67, 723-740.
- Murray, T., Yuan, Y., Thall, P., Elizondo, JH and Hofstetter, WL (2018) A Bayesian Utility-Based Stratified Medicine Design for the Effectiveness of Nutritional Prehabilitation in Thoracic Surgery, Biometrics, 74, 1095-1103.
- Chu, Y.* and Yuan, Y. (2018) A Bayesian Basket Trial Design Using Calibrated Bayesian Hierarchical Model. Clinical Trials, 15, 149-158.
- Shen, W*., Ning, J., Yuan, Y., Lok, A., Feng, Z. (2018) Model-free scoring system for risk prediction with application to hepatocellular carcinoma study, Biometrics, 74, 239-248.
- Liu, S., Guo, B. and Yuan, Y. (2018) A Bayesian Phase I/II Design for Immunotherapy Trials. Journal of the American Statistical Association, 113, 1016-1027.
- Murray, T.*, Thall, P. and Yuan, Y. (2018) A Bayesian Machine Learning Method for Optimizing Dynamic Treatment Regimes. Journal of the American Statistical Association, 113, 1255-1267.
- Riviere, M.K.*, Yuan, Y., Jourdan, J.H., Dubois, F. and Zohar, S. (2018) Phase I/II Dose-Finding Design for Molecularly Targeted Agent: Plateau Determination using Adaptive Randomization. Statistical Methods in Medical Research, 27, 466-479.
- Cai, C., Rahbar, M., Hossain, M., Yuan, Y. and Gonzales, N. (2017) A placebo-controlled Bayesian dose finding design based on continuous reassessment method in stroke research. Contemporary Clinical Trials Communications , 7:11-17.
- Yan, F., Mandrekar, SJ. and Yuan, Y. (2017) Keyboard: A Novel Bayesian Toxicity Probability Interval Design for Phase I Clinical Trials. Clinical Cancer Research, 23, 3994-4003.
- Chen, Z. , Li, Z, Zhuang, R., Yuan, Y., Kutner, M., Owonikoko, T., Curran, W. and Kowalski, J. (2017) Adaptive estimation of personalized maximum tolerated dose in cancer phase I clinical trials based on all toxicities and individual genomic profile, PLOS ONE, 12(1): e0170187.
- Guo, B. and Yuan, Y. (2017) Bayesian Phase I/II Biomarker-based Dose Finding for Precision Medicine with Molecularly Targeted Agents. Journal of the American Statistical Association , 112 , 508-520. ( Featured as JASA Applications and Case Studies – Invited Papers for 2018 JSM in Vancouver )
- Zhou, H.*, Lee, J. and Yuan, Y. (2017) BOP2: Bayesian Optimal Design for Phase II Clinical Trials with Simple and Complex Endpoints. Statistics in Medicine, 36, 3302-3314.
- Pan, H.* and Yuan, Y. (2017) A Calibrated Power Prior Approach to Borrow Information from Historical Data with Application to Biosimilar Clinical Trials. Journal of the Royal Statistical Society: Series C , 66, 979-996.
- Zang, Y.* and Yuan, Y. (2017) Optimal Sequential Enrichment Designs for Phase II Clinical Trials. Statistics in Medicine, 36, 54-66.
- Chu, Y.* and Yuan, Y. (2017) BLAST: Bayesian Latent Subgroup Design for Basket Trials Accounting for Patient Heterogeneity. Journal of the Royal Statistical Society: Series C , to appear.
- Chu, Y.* and Yuan, Y. (2017) A Bayesian Basket Trial Design Using Calibrated Bayesian Hierarchical Model. Clinical Trials, to appear.
- Liu, S., Guo, B. and Yuan, Y. (2017) A Bayesian Phase I/II Design for Immunotherapy Trials. Journal of the American Statistical Association , to appear.
- Murray, T.*, Yuan, Y. and Thall, P. (2017) A Bayesian Machine Learning Method for Optimizing Dynamic Treatment Regimes. Journal of the American Statistical Association , to appear.
- Yan, F., Mandrekar, SJ. and Yuan, Y. (2017) Keyboard: A Novel Bayesian Toxicity Probability Interval Design for Phase I Clinical Trials. Clinical Cancer Research, 23, 3994-4003.
- Zhou, H.*, Lee, JJ. and Yuan, Y. (2017) BOP2: Bayesian Optimal Design for Phase II Clinical Trials with Simple and Complex Endpoints. Statistics in Medicine , 36, 3302-3314.
- Pan, H.* and Yuan, Y. (2017) A Calibrated Power Prior Approach to Borrow Information from Historical Data with Application to Biosimilar Clinical Trials. Journal of the Royal Statistical Society: Series C , 66, 979-996.
- Guo, B.* and Yuan, Y. (2017) Bayesian Phase I/II Biomarker-based Dose Finding for Precision Medicine with Molecularly Targeted Agents. Journal of the American Statistical Association , 112, 508-520.
- Murray, T.*, Thall, P., Yuan, Y., McAvoy, S. and Gomez, D. (2017) Robust treatment comparison based on utilities of semi-competing risks in non-small-cell lung cancer. Journal of the American Statistical Association , 112, 11-23. ( Featured as JASA Applications and Case Studies – Invited Papers for 2018 JSM in Vancouver )
- Zang, Y.* and Yuan, Y. (2017) Optimal Sequential Enrichment Designs for Phase II Clinical Trials. Statistics in Medicine , 36, 54-66.
- Yuan, Y., Hess, K., Hilsenbeck, S. and Gilbert, M. (2016) Bayesian Optimal Interval Design: A Simple and Well-Performing Design for Phase I Oncology Trials. Clinical Cancer Research, 22, 4291-430.
- Yuan, Y., Guo, B, Munsell, M., Lu, K. and Jazzari, A. (2016) MIDAS: a practical Bayesian design for platform trials with molecularly targeted agents. Statistics in Medicine, 35, 3892-3906.
- Zang, Y.*, Liu, S. and Yuan, Y. (2016) Optimal Marker-strategy Clinical Trial Design to Detect Predictive Markers for Targeted Therapy. Biostatistics, 17, 549-560.
- Zhang, L.* and Yuan, Y. (2016) A Practical Bayesian Design to Identify the Maximum Tolerated Dose Contour for Drug Combination Trials. Statistics in Medicine, 35, 4924-4936.
- Riviere, M.K.*, Yuan, Y., Jourdan, J.H., Dubois, F. and Zohar, S. (2016) Phase I/II Dose-Finding Design for Molecularly Targeted Agent: Plateau Determination using Adaptive Randomization. Statistical Methods in Medical Research , to appear
- Chu, Y.*, Pan, H. and Yuan, Y. (2016) Adaptive dose modification for phase I clinical trials. Statistics in Medicine , 35, 3497-508.
- Iasonos, A. , Wages N., Conaway, M., Cheung, K., Yuan, Y. and O'Quigley, J. (2016) Dimension of Model Parameter Space and Operating Characteristics in Adaptive Dose-Finding Studies. Statistics in Medicine , 35, 3760-3775.
- Pan, H.* and Yuan, Y. (2016) A Default Method to Specify Skeletons for Bayesian Model Averaging Continual Reassessment Method for Phase I Clinical Trials. Statistics in Medicine, 36, 266-279.
- Zang, Y.*, J. Lee and Yuan, Y. (2016) Two-stage marker-stratified clinical trial design in the presence of biomarker misclassification. Journal of the Royal Statistical Society: Series C , 65, 585-601.
- Guo, B., Li, Y. and Yuan, Y. (2016) A dose-schedule-finding design for phase I/II clinical trials. Journal of the Royal Statistical Society: Series C , 65, 259-272.
- Chen, Z., Yuan, Y., Li, Z., Kutner, M., Owonikoko, T., Curran, W, Khuri, F. and Kowalski, J. (2015) Dose escalation with over-dose and under-dose controls in phase I/II clinical trials. Contemporary Clinical Trials , 43, 133-141.
- Shen, W.*, Ning, J. and Yuan, Y. (2015) Bayesian sequential monitoring design for clinical trials with non-compliance Statistics in Medicine , 34, 2104-2115.
- Zang, Y.*, Liu, S. and Yuan, Y. (2015) Optimal Marker-Adaptive Designs for Targeted Therapy Based on Imperfectly Measured Biomarkers. Journal of the Royal Statistical Society: Series C , 64, 635-650.
- Liu, S. and Yuan, Y. (2015) Bayesian Optimal Interval Designs for Phase I Clinical Trials. Journal of the Royal Statistical Society: Series C , 64, 507-523.
- Guo, B. and Yuan, Y. (2015) A Bayesian Design for Phase I/II Clinical Trials with Nonignorable Dropout. Statistics in Medicine , 34, 1721-1732.
- Shen, W.*, Ning, J. and Yuan, Y. (2015) A Direct Method to Evaluate the Time-dependent Predictive Accuracy for Biomarkers. Biometrics , 71, 439-449.
- Liu, S., Pan, H., Huang Q., Xia, J. and Yuan, Y. (2015) Bridging Continual Reassessment Method for Phase I Clinical Trials in Different Ethnic Populations. Statistics in Medicine, 10, 1681-1694.
- Riviere, M.K.*, Yuan, Y., Dubois, F. and Zohar, S. (2015) A Bayesian Dose-finding Design for Clinical Trials Combining a Cytotoxic Agent with a Molecularly Targeted Agent. Journal of the Royal Statistical Society: Series C , 64, 215-229.
- Jin, I.H.*, Liu, S., Thall, P. and Yuan, Y. (2014) Using Data Augmentation to Facilitate Conduct of Phase I/II Clinical Trials with Delayed Outcomes. Journal of the American Statistical Association , 109, 525-536.
- Riviere M.K., Yuan Y. , Dubois F. and Zohar S. (2014) A Bayesian dose-finding design for drug combination clinical trials based on the logistic model. Pharmaceutial Statistics, 13, 247-257.
- Zang, Y.*, Lee J. and Yuan, Y. (2014) Adaptive designs for identifying optimal biological dose for molecularly targeted agents. Clinical Trials, 11, 319-327.
- Cai, C.*, Liu, S. and Yuan, Y. (2014) A Bayesian Design for Phase II Clinical Trials with Delayed Responses Based on Multiple Imputation. Statistics in Medicine, 33, 4017-4028.
- Liu, S., Yuan, Y. , Castillo, R., Guerrero, T. and Johnson, V.E. (2014) Evaluation of Deformable Image Registration Spatial Accuracy Using a Bayesian Hierarchical Model. Biometrics, 70, 366-377.
- Cai, C.*, Yuan, Y. and Ji, Y. (2014) A Bayesian Phase I/II Design for Oncology Clinical Trials of Combining Biological Agents. Journal of the Royal Statistical Society: Series C , 63, 159-173.
- Liu, S., Yin, G. and Yuan, Y. (2013) Bayesian Data Augmentation Dose Finding with Continual Reassessment Method and Delayed Toxicity. Annals of Applied Statistics, 4, 2138-2156.
- Ahn, J.*, Yuan, Y., Parmigiani, G., Suraokar, M. B., Diao, L., Wistuba, I. and Wang W. (2013) DeMix: Deconvolution for mixed cancer transcriptomes using raw measured data. Bioinformatics, 29, 1865-1871.
- Cai, C.*, Yuan, Y. and Johnson, V.E. (2013) Bayesian adaptive phase II screening design for combination trials. Clinical Trials , 10, 353-362.
- Jin, I.H.*, Yuan, Y. and Liang, F. (2013) Bayesian analysis for exponential random graph models using the adaptive exchange sampler. Statistics and Its Interface, 6, 559-576.
- Zang, Y*. and Yuan, Y. (2013) A Shrinkage Method for Testing the Hardy-Weinberg Equilibrium in Case-Control Studies. Genetic Epidemiology , 37, 743-750.
- Yuan, Y., Thall, P. andWolf, J. (2012) Estimating Progression-free Survival When the Progression Status of Some Subjects is Unknown. Journal of the Royal Statistical Society: Series C , 61, 135-149.
- Yuan, Y. and Johnson, V.E. (2012) Goodness-of-Fit Diagnostics for Bayesian Hierarchical Models. Biometrics 68, 156-164.
- Huo, L.*, Yuan, Y. and Yin, G. (2012) Dose Finding in Drug Combinations with Discrete and Continuous Doses. Bayesian Analysis , 7, 235-252.
- Yuan, Y. and Yin, G. (2011) Robust EM Continual Reassessment Method in Oncology Dose Finding. Journal of the American Statistical Association 106, 818-831. ( featured article )
- Lei, X., Yuan, Y. and Yin, G. (2011) Bayesian phase II adaptive randomization by jointly modeling time-to-event efficacy and binary toxicity. Lifetime Data Analysis 17, 156-174.
- Yuan, Y. and Yin, G. (2011) Bayesian phase I/II drug-combination trial design in oncology. Annals of Applied Statistics , 5, 924-942.
- Yuan, Y. and Yin, G. (2011) Bayesian Hybrid Design in Phase I Oncology Clinical Trials. Statistics in Medicine, 30, 2098-2108.
- Yuan, Y., Huang, X. and Liu, S. (2011) A Bayesian response-adaptive covariate-balanced randomization design for clinical trials. Statistics in Medicine , 30, 1218-1229.
- Yuan, Y. and Yin, G. (2011) On adaptive randomization: is it useful? Journal of Clinical Oncology, 29, e390-e392
- Yin, G., Ma, Y., Liang, F. and Yuan, Y. (2011) Stochastic generalized method of moments. Journal of Computational and Graphical Statistics 20, 714-727.
- Yuan, Y. and Yin, G. (2011) Dose-response curve estimation: A semiparametric mixture approach. Biometrics 67, 1543-1554.
- Yin, G. and Yuan, Y. (2009) Bayesian Model Averaging Continual Reassessment Method in Phase I Clinical Trials. Journal of the American Statistical Association 104, 954-968.
- Song P.X., Li, M. and Yuan, Y. (2009) Joint Regression Analysis of Correlated Data Using Gaussian Copulas. Biometrics 65, 60-68.
- Yin, G. and Yuan, Y. (2009) A latent contingency table approach to dose-finding for combinations of two agents. Biometrics 65, 866-875.
- Yin, G. and Yuan, Y. (2009) Bayesian Dose-finding in Oncology for Drug Combinations by Copula Regression. Journal of the Royal Statistical Society: Series C 58, 211-224.
- Yuan, Y. and Yin, G. (2009) Bayesian Dose-finding by Jointly Modeling Toxicity and Efficacy as Time-to-Event Outcomes. Journal of the Royal Statistical Society: Series C 58, 719-736.
- Yuan, Y. and Johnson, V.E. (2008) Bayesian Hypothesis Tests Using Nonparametric Statistics. Statistica Sinica 18, 1185-1200
- Yuan, Y. and Yin, G. (2008) Sequential Continual Reassessment Method for Two-dimensional Dose Finding. Statistics in Medicine 27, 5664-5678.
-
Missing Data Analysis
- Ahn, J.*, Liu, S., Wang W. and Yuan, Y. (2013) Bayesian Latent-class Mixed-effect Hybrid Models for Dyadic Longitudinal Data with Non-ignorable Dropouts. Biometrics, 69, 914-924.
- Zhang G. and Yuan, Y. (2012) Modeling Longitudinal Dyadic Data with Nonignorable Dropout with Application to a Breast Cancer Study. Annals of Applied Statistics, 6, 753-771.
- Yuan, Y. and Yin, G. (2010). Quantile regression for longitudinal studies with nonignorable missing data. Biometrics, 66, 105-114.
- Yuan, Y. and Little, R.J.A. (2009) Meta-analysis of studies with missing data. Biometrics 65, 487-496.
- Yuan, Y. and Little, R.J.A. (2009) Mixed-effect hybrid models for longitudinal data with nonignorable dropout. Biometrics 65, 478-486. Yuan, Y., and Little, R.J.A. (2007) Model-Based Estimates of the Finite Population Mean for Two-Stage Cluster Samples with Unit Non-response. Journal of the Royal Statistical Society: Series C 56, 79-97.
- Yuan, Y., and Little, R.J.A. (2007) Parametric and Semiparametric Model-based Estimates of the Finite Population Mean for Two-Stage Cluster Samples with Item Nonresponse. Biometrics 63, 1172-1180.
-
Mediation Analysis
- Huang, J.* and Yuan, Y. (2016) Bayesian Dynamic Mediation Analysis, Psychological Methods, to appear.
- Yuan, Y. and MacKinnon D. (2014) Robust mediation analysis based on mediation regression. Psychological Methods, 19, 1-20.
- Yuan, Y. and MacKinnon D. (2009) Bayesian mediation analysis. Psychological Methods, 14, 301-322.